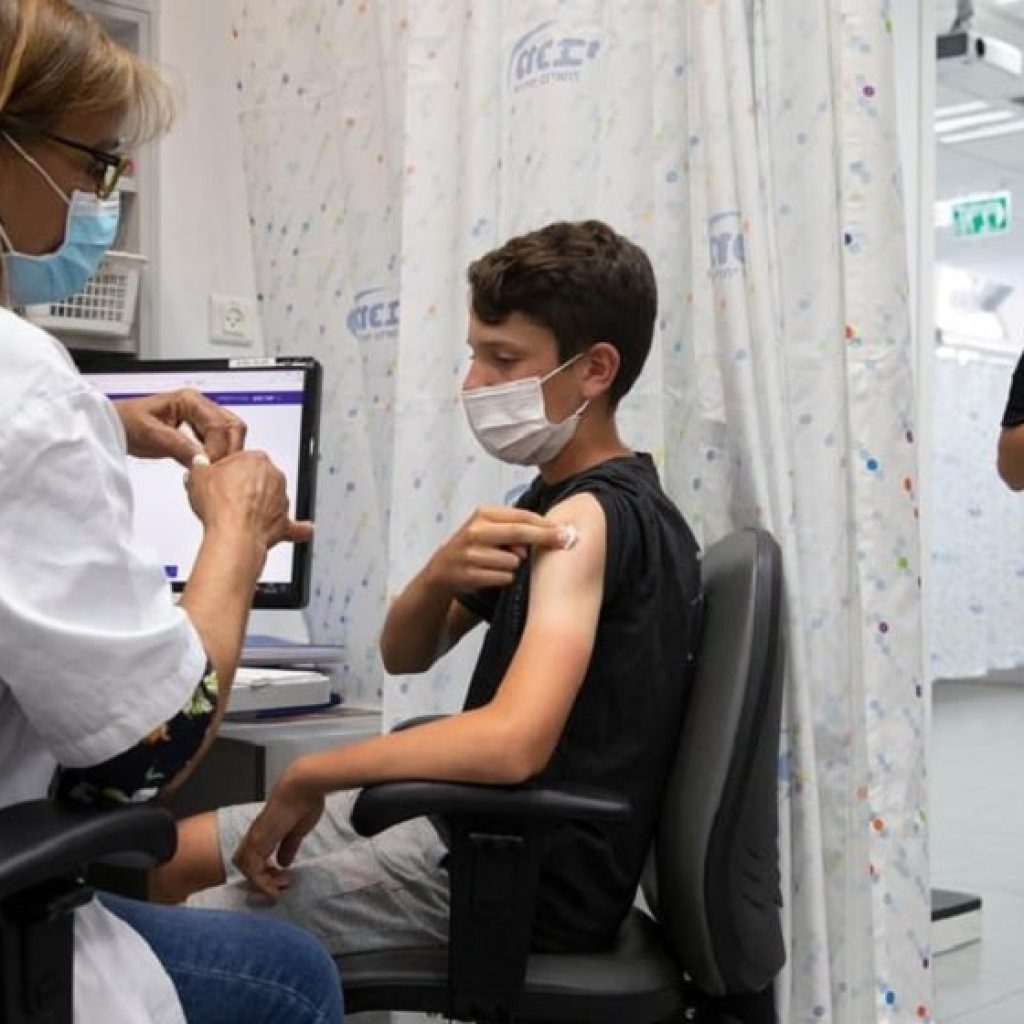
Pfizer Inc. and BioNTech SE’s COVID-19 vaccine will be tested using lower doses on children under the age of 12 in an earlier stage of the trial.
The vaccine partners announced that 4,500 children will be registered at more than 90 clinical sites in the United States of America, Finland, Poland, and Spain to test the vaccine to determine if COVID-19 protection can be extended to all ages.
ALSO READ
NCOC Makes a Big Decision on COVID-19 Restrictions
Children between the ages of five and eleven are being registered for the study. Pfizer will test a dose of 10 micrograms on them, and three micrograms of the dose on children between the ages of six months and five years based on the findings from an early-stage trial that had evaluated the safety, tolerability, and immune response generated by the vaccine.
Canada, the US, and the European Union have already authorized the emergency use of the Pfizer vaccine for individuals aged 12 and above.
ALSO READ
Govt to Spend $1 Billion to Import More COVID-19 Vaccines
Michael Joseph Smith, a pediatric infectious disease specialist and the co-principal investigator of a Duke University-based trial site said, “Kids are not little adults. The younger you are, the more robust your immune system is and the more of a reaction you have, so essentially, you can get by with less antigen but get the same immune response without high fever or swollen arms”.
The post Pfizer Vaccine to be Tested on Kids appeared first on .